Electron Relay Systems
Background
|
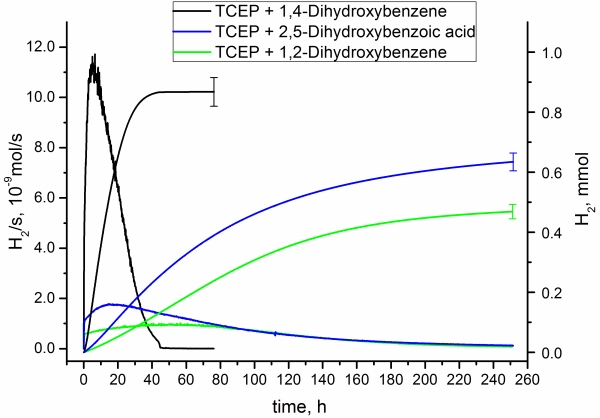
In the natural photosystem, the oxidative and the reductive branch are coupled by hydroquinone/quinone system, which carries the electrons from water oxidation (PSII) to the site of “proton reduction” (PSI). In artificial systems, the two sites are often separated and an electron donor replaces PSII or an electron acceptor PSI respectively. For proton reduction, ascorbic acid and tertiary amines are most frequently used as sacrificial electron donors (SED). We study in detail system in which theses SEDs could act as true, reversible relays rather than just SEDs. The idea is to find a molecular couple with correctly aligned orbitals for mimicking the function of plastoquinone in the natural system. A particular focus is put on quinones. We showed, that ascorbic acid in the presence of a phosphine transforms from pure SED to a real electron transfer relay. Recently, we extended this concept and showed for the first time a system using hydroquinones as electron relays. |
Figure 1. Performances of different hydroquinones as electron relays between TCEP as an SED and the proton reduction cycle |
Challenges
Coupling the proton reduction system to water oxidation – elucidation of the mechanism and the elementary steps – finding of energetically aligned relays – triad systems
|
|
|
||
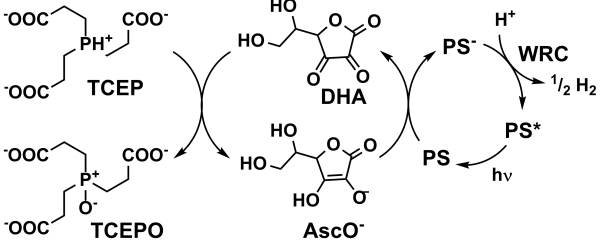
| Scheme 5. Ascorbic acid as an electron relay rather than a sacrificial electron donor, the TCEP phosphine represents the ultimate SED | |||
Coworkers
Mathias Mosberger, Margherita Orazietti, Johannes Windisch, Nico Weder, Peter Müller (PhD students), Dr. Benjamin Probst
Selected Publications
- Ascorbate as an electron relay between an irreversible electron donor and Ru(II) or Re(I) photosensitizers.Bachmann, C., Probst, B., Guttentag, M. and Alberto, R. (2014): Chem. Commun. 50, 6737-6739.
- Quinones as Reversible Electron Relays in Artificial Photosynthesis.Rodenberg, A., Orazietti, M., Mosberger, M., Bachmann, C., Probst, B., Alberto, R. and Hamm, P. (2016): ChemPhysChem 17, 1321-1328.