_____________________________________________________________________________________________________________________

Background
|
Scheme 1. Typical reaction protocol for the ones step synthesis |
This core represents our entry into bioorganometallic chemistry, although it is still a debate if CO complexes are organometallic. The full complex [99mTc(OH2)3(CO)3]+, a kind of organometallic aquo-ion, is prototypical for the request for a building block in radiopharmaceutical chemistry. It is prepared in >98% yield in one step from [99mTcO4]- and in water. The three water ligands are labile and can be substituted with many ligands to yield highly inert closed-shell complexes. If the ligand is attached to a targeting molecule, labeling is enabled. Numerous groups worldwide used this approach and one compound is |
| currently in phase III clinical trials. Whereas the 99mTc complexes allowed labelling, the 99Tc analogue which preparation was recently described in Inorg. Syn. Vol 36 enabled the study of subsequent organometallic chemistry without the need of performing high-pressure high-temperature autoclave reactions. | |
.2016-11-23-16-00-01.gif) |
|
|
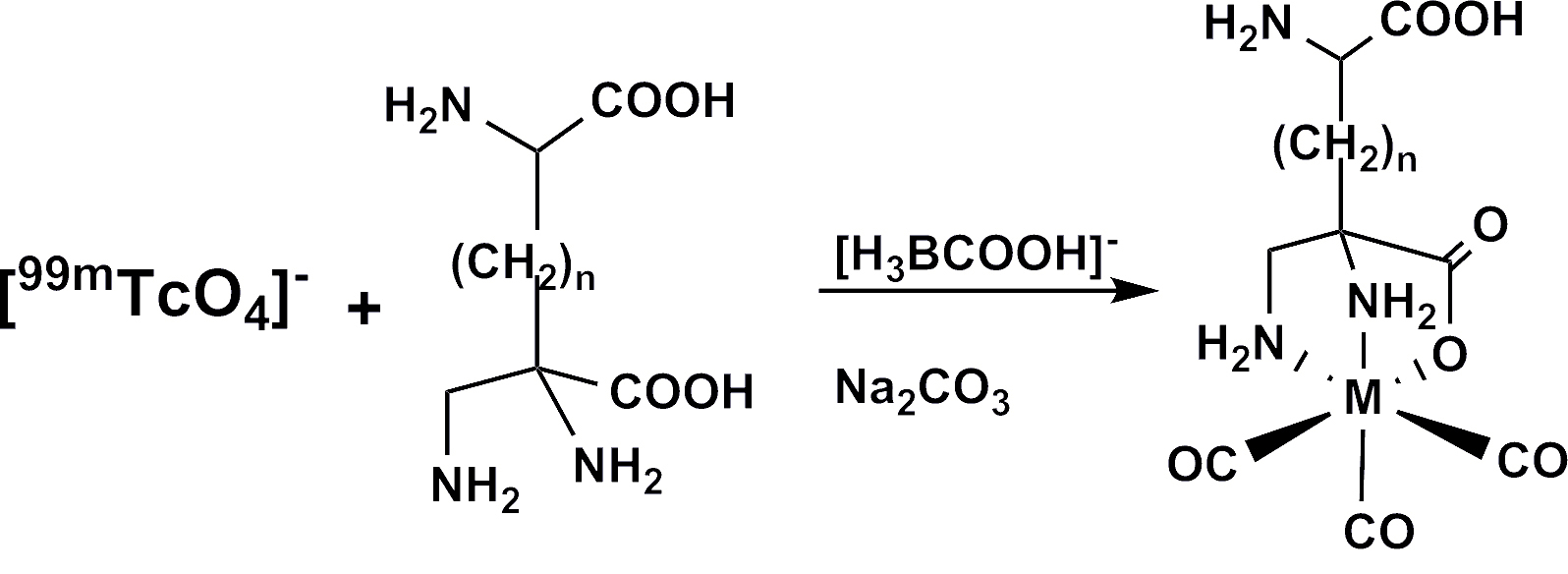
| Figure 1. ORTEP presentation of [99Tc(2,3-dap)(CO)3], one of the most stable and smallest complex with the {Tc(CO)3}+ core, used for labeling artificial amino acids. |
Figure 2.ORTEP presentation of [ReBr2(CO)4]- |
Current research
Although the basics around this core are known, we are still developing ligands, which are more efficient in terms of rate of formation than the best ones we have and as small as possible, adaptable to the biological properties of the particular targeting core. We also use the core for mechanistic studies. Recently, we studied the kinetics of CO exchange, which revealed a very interesting and unexpected mechanism.
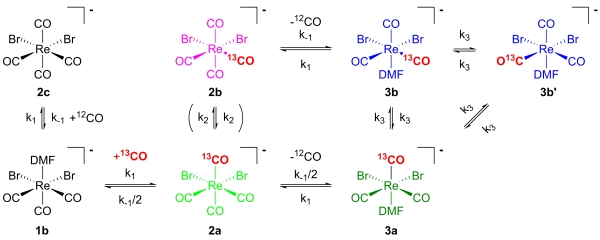
Scheme 2. Steps of 13CO exchange in [ReBr2(dmf)(CO)3]+.
Challenges
Prepare [99mTc(OH2)3(CO)3]+ at room temperature – replace CO ligands with solvents – find highly efficient ligands.
Coworkers
Angelo Frei in collaboration with Prof. Andreas Roodt, University of the Free State, South Africa
Collaboration
Prof. Andreas Roodt, University of the Free State, South Africa (Swiss South Africa Joint Research Project)
Publications
Amino acids labeled with [99mTc (CO)3]+ and recognized by the L-type amino acid transporter LAT1. Liu, Y., Pak, J. K., Schmutz, P., Bauwens, M., Mertens, J., Knight, H. and Alberto, R. (2006). J. Am. Chem. Soc. 128, 15996-15997.
Kinetics and Mechanism of CO Exchange in [MBr2(solvent)(CO)3]- (M=Re, 99Tc).Frei, A., Sidler, D., Mokolokolo, P., Braband, H., Fox, T., Spingler, B., Roodt A. and Alberto, R. (2016): Inorg. Chem., 55, 9352–9360
4.2016-10-13-16-09-06.jpg)